An Oxidation-Reduction Reaction Essay
An Oxidation-Reduction Reaction Essay
Question description
Which of the following chemical reactions is an oxidation-reduction reaction? CuSO4 + BaCl2 yields BaSO4 + CuCl2 AgNO3 + NaCl yields AgCl + NaNO3 Pb(NO3)2 + 2NaCl yields PbCl2 + 2NaNO3 Zn + H2SO4 yields ZnSO4 + H2. An Oxidation-Reduction Reaction Essay
ORDER A PLAGIARISM-FREE PAPER HERE
Which of the following describes an element that is oxidized in a reaction? (2 points)
| It gains electrons during a reaction. | |
| It has a decrease in its oxidation number. | |
Al 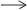 Al3+ + 3e− Al3+ + 3e− |
|
F2 + 2e− 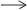 2 F− 2 F− |
5.
(07.05 LC)
Which of the following describes an element that is reduced in a reaction? (2 points)An Oxidation-Reduction Reaction Essay
| It acts in the reactant that is the reducing agent. | |
| It has a decrease in its oxidation number. | |
Cd 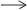 Cd2+ + 2e− Cd2+ + 2e− |
|
Zn + Cu2+ 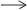 Zn2+ + Cu Zn2+ + Cu |
6.
(07.05 MC)
Consider the following reaction:
Cl2 + 2NaBr 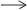 2NaCl + Br2
2NaCl + Br2
Which element is oxidized and which is reduced in the reaction? (2 points)
| Br is reduced and Na is oxidized. | |
| Cl is reduced and Br is oxidized. | |
| Cl is reduced and Na is oxidized. | |
| Na is reduced and Br is oxidized. |
An Oxidation-Reduction Reaction Essay
